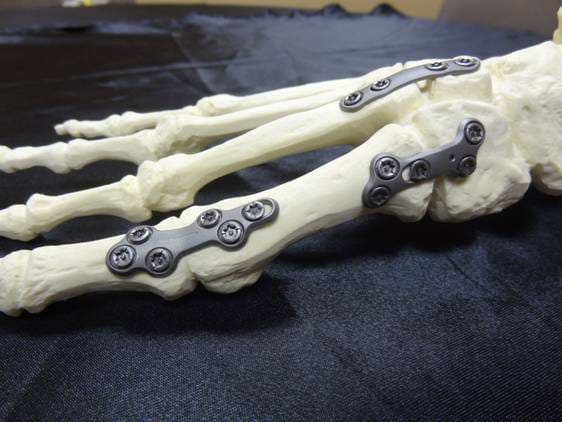
Our team is dedicated to providing innovative solutions to the orthopedic community. With over twenty years of experience in orthopedic design and development, we provide startup and large orthopedic companies the expertise they need from concept through commercialization. Our team utilizes the latest 3D software combined with finite element analysis (FEA) to verify designs and ensure that prototypes function per our customer requirements. Our dedication and commitment to on time delivery ensures our customers timelines are always met.
We believe in concurrently working with our manufacturers during the development process. This ensures that our designs are not only innovative and unique, but can also be made simply and easily. Our team works with manufacturers to create 3D models and drawings which can be utilized in both rapid prototype and production machines. This technique speeds up the development cycle, eliminating months from our customers timeline while saving them money.
We believe in concurrently working with our testing partners from early on in the development cycle. This ensures that our designs will pass testing with a high success rate. Reducing risk of failure in testing keeps original timelines on target.
Intrepid Orthopedics offers medical device regulatory consulting. With over 20+ years of experience, we have the knowledge to guide you through your regulatory pathway. We can help you whether you are seeking regulatory approval for a Class I, II or III device or responding to observations and warning letters.